PLUTONIUM:
THE LAST FIVE YEARS
Part I:
The Trouble With Plutonium
A Review of Plutonium
Destructiveness, Complexity, and Hazards i
Plutonium will be with us for a long time, and
not only because it has a radioactive half-life
of 24,000 years and therefore is dangerous for
more than 200,000 years. Plutonium will be with
us because nuclear weapon states are deeply
devoted to having it as a military presence, the
global nuclear power establishment is deeply
devoted to pushing it as the fuel of the future,
and the personal and political opinions of
scientists often carry more weight than their
scientific opinions.
A passage from the most recent issue of Los
Alamos Science, No. 26-which is must reading
for plutonium foes and friends alike-illustrates
this reality:
“Regardless of popular or
political opinions about the uses of
plutonium, plutonium processing will
continue globally at least for many
decades. In the United States, plutonium
plays a central role in national defense;
it is routinely formed into samples for
experiments, cast or machined into
nuclear weapon pits, and extracted from
retired nuclear weapons or weapon
components and prepared for disposal. All
of these activities require that
plutonium be chemically or mechanically
processed.” ii
|
| Nightmare or
Dream? |
| “Plutonium is a
physicist’s dream but an
engineer’s nightmare. With little
provocation, the metal changes its
density by as much as 25 percent. It can
be brittle as glass or as malleable as
aluminum; it expands when it solidifies,
much like water freezing to ice...it is
highly reactive in air...plutonium
damages materials on contact and is
therefore difficult to handle, store, or
transport. Only physicists would ever
dream of making and using such a
material. And they did make it-in order
to take advantage of the extraordinary
nuclear properties of
plutonium-239.” Plutonium,
An Element at Odds with Itself. Los
Alamos Science. 2000. Number 26. |
This emphasis on the military use of plutonium
suggests that without the military applications,
support for “peaceful uses” of
plutonium 239 would be meager. Plutonium may be a
nuclear weapons physicists’ dream (see
sidebar), but the dreams of physicists do not
always come true, as is evident in the case of
the now defunct Superconducting Super Collider
project of the 1980's.
So while the pro-plutonium inertia is powerful,
it is not omnipotent and the future of this
element and other special nuclear weapons
materials is not set in stone. As the debate
continues to unfurl, it is important for people
to know that this most secret of elements is the
most complex metal in the periodic table; and its
presence in deployed nuclear weapons threatens
life as we know it.
Plutonium
in Nuclear Explosives
Plutonium-239 is a fissile material well-known
for its use as the primary trigger in most
nuclear explosives (Figure 1-1). All grades of
plutonium (see Table 2-1) are considered useable
in nuclear explosives, but weapon-grade
plutonium--which contains more than 92%
plutonium-239--is preferred for nuclear weapon
arsenals because lower amounts of plutonium-239
found in fuel and reactor grade pose a much
higher risk of “pre-initiation” of the
trigger due to corresponding higher amounts of
plutonium-240. Use of lower grades also makes
fabrication of the plutonium trigger, or pit,
more difficult.iii
Because of its use in weapons of mass
destruction, plutonium accounting is conducted to
the level of grams, and large security forces are
necessary to guard it.
However, the use of fuel or reactor grade
plutonium is considered an easier path for a
nonweapons state or a terrorist group because:
easiest way to make a nuclear weapon is with
reactor-grade plutonium because:
there is much more of it in the world,
approximately 1300 metric tonnes in
irradiated nuclear fuel, and another xx
MT separated and awaiting use as reactor
fuel.
it does not require the use of a
“neutron generator.” As the
Department of Defense puts it, “ a
nuclear device used for terrorism need
not be constructed to survive a complex
stockpile-to-target sequence, need not
have a predictable and reliable yield,
and need not be efficient in its use of
nuclear material.”iv
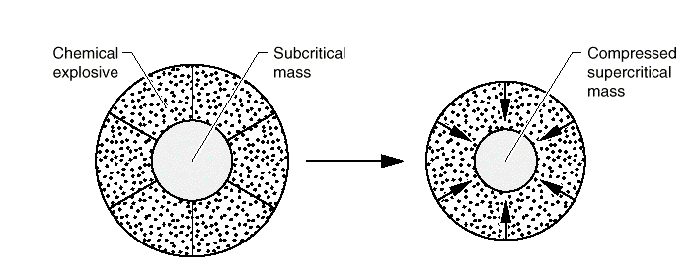
Figure 1-1. A
simplified illustration of how a precise
detonation of chemical high explosives
surrounding a subcritical mass of fissile
materials generates enough force to initiate, or
trigger, the nuclear detonation. Source: Los
Alamos Science. Number 23. 1993. Page 55.
Plutonium
Chemical Complexity
If anything contributes to plutonium’s
demise as a military tool it will be its inherent
chemical instability. The future of the plutonium
triggers in the U.S. nuclear weapons stockpile is
the focus of intense debate both internally and
externally to the weapons labs and in the
Pentagon. In particular, the lack of
understanding of how plutonium ages is driving
calls for renewed large-scale pit production.
Lawrence Livermore National Laboratory spins it
this way, “predicting kinetics is crucial to
avoiding surprise requirements for large-scale
refurbishment and remanufacture of weapons
components.”v
| Baffled
Scientists |
“We conclude that the
present understanding of plutonium
chemistry is inadequate and that the new
evidence presents an immediate challenge
to the scientific community.”
Hascke, Allen, and Morales. Surface and
Corrosion Chemistry of Plutonium.
|
“The bad news is that
plutonium is very complicated...we
actually don’t know how aged
plutonium.”
Dr. Bruce Tarter, Director of Lawrence
Livermore National Laboratory.
|
Delta-phase plutonium-gallium
alloy is the “most useful and
familiar phase [but] the least understood
theoretically.” Sig Hecker,
Los Alamos National Laboratory.
|
“Seaborg had the choice of
picking the symbol Pl or Pu for
plutonium. He remarked that it is really
kind of a stinky element (complicated
chemistry and unusual metallurgical
properties) so it became Pu.”
R.H. Condit. Plutonium. An Introduction.
|
Plutonium is cited by the nuclear weapons labs as
the most complex metal in the periodic table and
continues to baffle people who best understand it
(see sidebar). U.S. and Russian weapons
scientists do not even agree on the “phase
diagram” for the easily machinable
delta-phase plutonium that dominates nuclear
weapons stockpiles.vi
Its traits are commonly described as
unstable, unpredictable, anomalous, and
dramatically variable in the open literature. The
litany of difficulties includes:
an inherent instability marked by adverse
reactivity as a metal or an oxide powder
with common items like air, water, and
oils, which also “makes it difficult
to keep track of plutonium
inventories.”vii
corrosion from hydrides and oxides from
the outside-in and from radioactive decay
from the inside-out;
runaway corrosion reactions;
an ability to cling
“tenaciously” to anything and
everything;viii resulting in buildups of
plutonium in ductwork, piping, and
ventilation systems;
ultra-sensitivity to temperature and
pressure changes, with marked increases
in density with phase changes (Figure
1-3);
an “anomalously low melting
point;”
pyrophoricity: spontaneous ignition at
certain temperatures and certain particle
sizes.
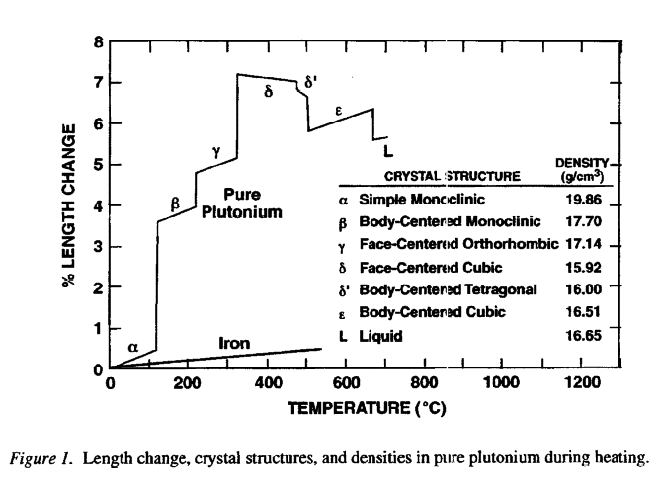
Figure 1-2. This diagram is
commonly used to illustrate plutonium complexity,
showing the contrasts between the dramatic and
abrupt six phase changes of plutonium as it is
heated compared to the stability of iron. Some of
the key traits of the different phrases include:
· Alpha-phrase plutonium is brittle and
difficult to machine, like cast iron.
· Small amounts of aluminum alloyed with
delta-phase plutonium stabilize the plutonium and
produces a metal as machinable as aluminum.
However, because aluminum emits neutrons upon
absorbing alpha particles from the decay of
plutonium, it raises the risk of pre-initiation,
or early criticality, of the plutonium trigger.
· Gallium alloyed with delta-phase plutonium
retains the benefit of a product nearly
machinable as aluminum and far less prone to
plutonium oxidation without raising the risk of
pre-initiation, and therefore the
plutonium-gallium alloy is the most common in
plutonium pits.
To make plutonium fuel, DOE intends to
destabilize plutonium by removing gallium during
purification.
Plutonium Hazards
| “Many opportunities
exist for mistakes in working with
plutonium chemistry...The penalties for
mistakes include spills of radioactive
materials and possibly criticality
experiments.” |
R.H. Condit. Introduction to Plutonium. |
The combination of radioactivity and chemical
instability makes plutonium in the workplace an
inherently unsafe enterprise even after it is
produced and separated.
Add to this the need for precise accounting to
the gram level and large protective forces to
guard vaults and other storage areas, and the
costs of dealing with plutonium become
exorbitant.
Primary among the numerous aspects of the
plutonium radiation hazard is the fact that it
takes 24,400 years for it to lose one half of its
radioactivity, meaning that it will remain
dangerous for hundreds of thousands of years and
react adversely when exposed to common
environments.
Alpha
Radiation and Decay
Plutonium-239 emits high levels
of alpha radiation (Figure 1-3). Although alpha
radiation can be stopped with paper, it causes
damage in many ways and from several phenomenon.
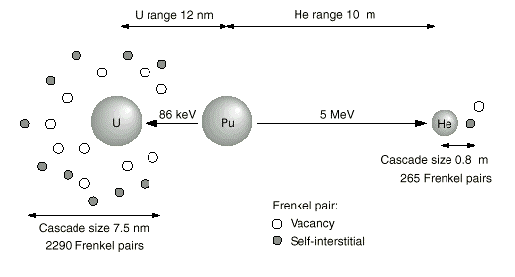
Figure 1-3. The first part of the
plutonium-239 decay chain. Plutonium decays to
Uranium 238 by emitting an alpha particle, in
this case a helium nucleus. The energy from this
process drives several reactions that are poorly
understood.
Source: Los Alamos Science. Number 26. 2000.
1. Damage to the plutonium over time.
The recoil energy from the decay generates 85
kilo-electron-volts of kinetic energy in the
uranium nucleus, of which 60 keV remains when the
nucleus collides within the matrix and displaces
plutonium atoms in the metal.ix Over the course
of decades, this action can damage plutonium
enough to keep weapons designers leery of the
“reliability” of the plutonium
triggers.
The helium nucleus has far more energy when
released, 5 million-electron-volts, but this is
said to lose all but 0.1 percent of its energy
through collisions with electrons before
capturing a few electrons and “settling
in” as a helium atom.x Over the course
of decades, helium atoms accumulate to the point
of creating bubbles, another grave concern of
weapons designers. Helium buildup also poses a
health and safety risk. For example, in 1963 a
plutonium pit tube broke during a weapon
disassembly process at Pantex and contaminated
workers and the facility with plutonium
contaminated helium gas.
2. Damage to other metals over time.
Plutonium decay basically damages everything in
its path, and this impact is most measurable on
elements that experience “void
swelling” from radiation, meaning they swell
in size over time.xi The effects of
this over the course of decades is poorly
understood because plutonium has never been
allowed to age for decades, but some implications
are obvious:
Beryllium, which is used
as a neutron tamper within pits and as
cladding on many plutonium pits (see Part
III) serving to protect the plutonium
from oxidizing, experiences
“gas-driven” swelling;.
Aluminum, which is used
in cladding on some pits, suffers from
void swelling.
Iron, Chromium, and
Nickel, the key ingredients in stainless
steel used for plutonium storage cans,
experiences void swelling;
Zirconium, used to clad
nuclear fuel, experiences void swelling.
3. Damage to live
tissues. If the uranium nuclei from
decay damages metal as dense as plutonium, the
impacts on living tissue are quite obvious.
Plutonium is said to be “harmless” if
ingested as a metal, but this is an obvious
fallacy since even plutonium metal has a layer of
plutonium oxide present at all times,xii oxides are
always present to some degree on metals, and the
chemical reactions with common materials that
worry metallurgists and weapons designers are
certainly a concern inside the human body.
Plutonium is most hazardous in a powder form.
Much debate has occurred over how much plutonium
oxide can cause lung cancer within a few decades,
with estimates ranging from a few micrograms to
30-60 micrograms to 2 milligrams. There seems to
be little debate over how much will kill a
person:
Ingestion of 500
milligrams, or one half of a gram, is
considered the acute lethal dose;
Inhalation of 20
milligrams is considered the acute lethal
dose;xiii
A good scale for reference is a
typical Sweet N’ Low packet which contains
one million micrograms of sugar substitute.
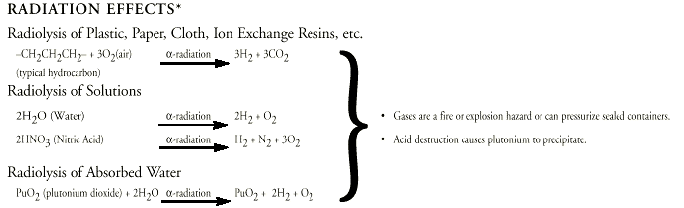
4. Radiolysis of common
materials. Alpha particles react with
materials such as air and water to cause
“radiolysis” of common materials
(Figure 1-4). Plutonium metal oxidizes readily in
air and plutonium oxide generates gases that can
rupture storage containers. Plutonium is most
hazardous in a powder form.
Figure 1-4. Simplified
illustration showing various reactions brought
about by alpha decay.
Source: Los Alamos Science. Number 26.
The literature is filled with reports about
ruptured containers and massive oxidation of
entire metal pieces. For example, in 1983 Los
Alamos reported the formation of a black powdered
suboxide in “casting skulls” left over
from plutonium pit fabrication, and when
containers of skulls were opened, the plutonium
suboxide would ignite “almost
explosively.”xiv
To avoid these undesirable reactions, DOE finally
established a long-term storage standard for
plutonium in 1994, but has had trouble meeting
that standard (see Part II, Section B.) Called
the 3013 standard, it requires that plutonium
metals and oxides be stored in two sealed metal
containers free of organic materials. Reaching
this standard requires heating of oxides to
temperatures greater than 900 degrees Celsius.
A few near-term implications of this chemical
fact include:
1. Nitric acid processing, which DOE plans to use
to purify plutonium oxide as the first step
towards making plutonium MOX, greatly increases
the likelihood of explosions, spills, and
criticality events. The plutonium pit disassembly
and conversion facility is planned as the main
source of plutonium oxide for a plutonium fuel
(MOX) factory. Early plans for the PDCF require
the plutonium oxide product to meet the long term
plutonium storage (3013) standard.xv
2. The dangers of nitric acid plutonium
processing are aggravated if the plutonium oxide
was produced or treated at temperatures greater
than 600 degrees Celsius. Oxides heated to
temperatures between 600 and 1000 C “require
somewhat more stringent procedures” when
dissolving in acids, and plutonium oxide powder
heated to temperatures over 1000 Celsius
“require extreme measures.”xvi
Since the long-term storage
standard requires plutonium to be heated at
temperatures well above 600 degrees C,xvii it is
incompatible with the needs of plutonium fuel
production.
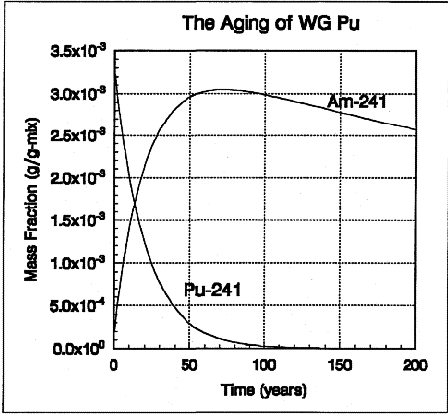
Aging
Plutonium and Americium-241
Plutonium-241, which is present in all grades of
plutonium, decays into the more radioactive and
dangerous americium-241, an intense gamma ray
emitter that is 100 times more toxic than
plutonium 239. Weapons plutonium was routinely
purified to eliminate americium, which of course
produced stockpiles of americium. If plutonium
decay is allowed to run its course, radiation
levels in U.S. plutonium will peak in the next 38
to 60 years (Figure 1-4).
Figure 1-4. As plutonium-241
decays to Americium-241, weapon grade plutonium
becomes more hazardous and radioactive. Americium
levels peak after 70 years. Source: Peterson,
1993. RFP-4910.
|